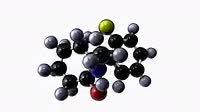
Copper flame test. A sample of copper chloride is atomised and sprayed into a Bunsen burner flame. The copper ions (Cu2+) absorb energy from the flame and the electrons of the copper ions make transitions between energy levels. Copper ions in excited states can transition to lower energy levels and this accompanied by the emission of light of specific wavelengths. For copper, the emission of green wavelengths of light (532 nm) are seen.
Details
WebID:
C01808657
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:20.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No











 Loading
Loading