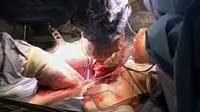
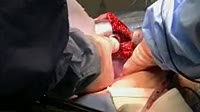
Demonstration of the voltage generated by a zinc-bromine galvanic cell. The beakers contain a solution of zinc bromide (left) and liquid bromine (right), linked by a salt-saturated bridge of wet paper. A voltmeter is connected to two electrodes, of zinc metal in the zinc solution and graphite in the bromine. When the electrode is placed in the bromine, a voltage of 1.67V is seen on the voltmeter. This is produced as the highly electronegative bromine attracts electrons from the zinc, turning the metal into zinc ions, which enter solution. The bromine itself is reduced to bromide ions. This generates a flow of electrons and ions around the circuit. The zinc-bromine cell is used in industry as a rechargeable storage battery, as it can be charged and discharged thousands of times.
Details
WebID:
C01787529
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:12.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
No
Property Release
No











 Loading
Loading