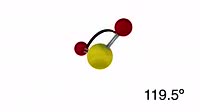
Animation of a model of a molecule of hydrogen fluoride (HF), showing its polarity. Polarity is a property of a molecule that has an uneven distribution of electric charge around it, giving it two or more poles of opposite charge. Here, red areas have a slight negative charge, blue ones a positive charge. This arises as the fluorine atom has a three lone pairs of negative electrons that are not involved in bonding, and also attracts the bonding electrons away from the hydrogen atom as well, leaving it with a small positive charge. The measure of an atom's ability to attract electrons is called its electronegativity, and is related to the number of protons it has in its nucleus, and the number of electron shells around the nucleus. Polarity plays an important part of determining the properties of a substance. With an asymmetrically-charged molecule like hydrogen fluoride, the negative side of one molecule can attract and form weak temporary bonds with the positive side of a nearby molecule. At a macroscopic level means the substance has a higher melting and boiling point that may be expected. It also means that the substance can dissolve or dissolve in other polar or charged substances. Hydrogen fluoride is completely miscible in water, for instance. Compare this situation with a molecule of hydrogen (H2), in clip K004 3631.
Details
WebID:
C01787170
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:10.000
Format:
QuickTime
Bit Rate:
24 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No










 Loading
Loading