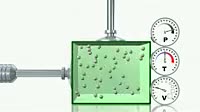
Animation showing Boyle磗 law in an ideal gas. The dials at right show the pressure P, temperature T and volume V of the gas. Boyle磗 law states that, at a constant temperature, a decrease in the volume of a gas causes a proportional increase in pressure. When the arms compress the box, the particles of gas grey collide far more frequently with the sides of the container, which is seen as increased pressure. The law is named after Robert Boyle 1627_1691, who first published it in 1662. This animation is part of a series on the ideal gas laws. See K003 3525_3530 for the complete set.
Details
WebID:
C00617591
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
000:30.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
NO












 Loading
Loading