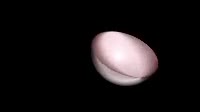
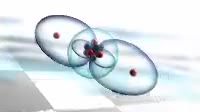
Animation of the structure of the 1s atomic orbital, cut in half to show the internal structure. Orbitals contain an atom磗 electrons, which are found around the central atomic nucleus not seen. Each orbital can hold two electrons. The 1s orbital is the first spherical s orbital out from the nucleus, making it the lowest energy of all orbitals. Orbitals further from the nucleus are at a higher energy, and orbitals are filled from the lowest energy level one first. Therefore the 1s orbital is full for every element heavier than hydrogen. An atomic orbital is a mathematical model of the location of an electron around an atomic nucleus. Electrons do not orbit the nucleus as might be imagined, but instead exist as a standing wave around it. The 1s orbital only has one phase. Higher energy s orbitals have concentric shells of positive and negative wave function.
Details
WebID:
C00607280
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
000:18.000
Format:
QuickTime
Bit Rate:
24 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No











 Loading
Loading