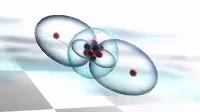
Animation of the bonding in a beryllium hydride BeH2 molecule. Initially a beryllium atom has two populated electron orbitals, an inner spherical 1s shell blue and an outer spherical 2s shell grey. Beryllium typically forms ionic compounds through the loss of the 2s electrons, but it can also form covalent compounds with hydrogen. In beryllium hydride, one 2s electron red and white rises to a vacant 2p level red, and the 2p orbital and the 2s orbital hybridise, producing two identical sp orbitals dark blue, arranged pointing away from each other. These then bond with hydrogen atoms, with a release of energy flash.
Details
WebID:
C00607258
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
000:31.000
Format:
QuickTime
Bit Rate:
24 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No









 Loading
Loading