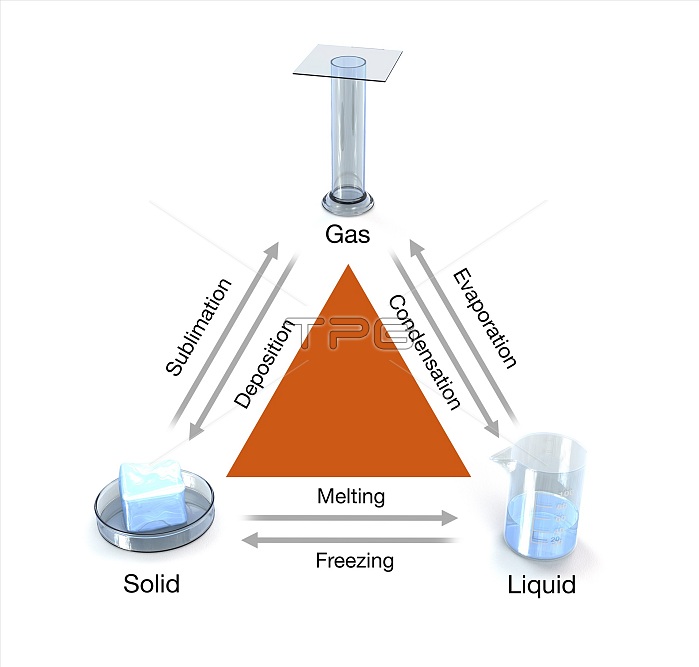
States of matter. Computer illustration showing the three states of matter and how any one of them can be converted (arrows) directly into one of the other two - by an increase or reduction of energy in the form of heat. Clockwise from: gas, liquid and solid. Molecules are in constant motion. In solids, the molecules have a strong attraction, are close together and have limited motion. In a liquid, the molecules have more energy, which enables them to overcome some of the attraction and move more freely. In a gas, attraction between particles is minimised and they move freely throughout the container.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP15939339
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
No
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading