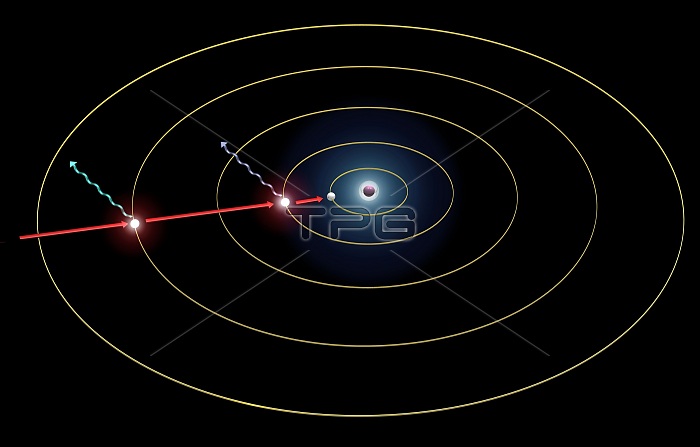
Hydrogen spectrum emission levels. Diagram showing the quantum basis for the emission spectrum of a hydrogen atom. When electrons in an element become excited (by heating), they enter higher energy orbits. When they return to their ground state they release the extra energy as light radiation at a specific wavelength characteristic of that element. Shown here (electrons in their excited states) are: the 4-2 transition, emitting blue-green light at 486.1 nanometres, part of the Balmer series; and the 2-1 transition, emitting ultraviolet light at 121.5 nanometres, part of the Lyman series. For this diagram with labels, see C025/8084.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP15513301
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
No
Property Release:
No
Right to Privacy:
No
Same folder images:

 Loading
Loading