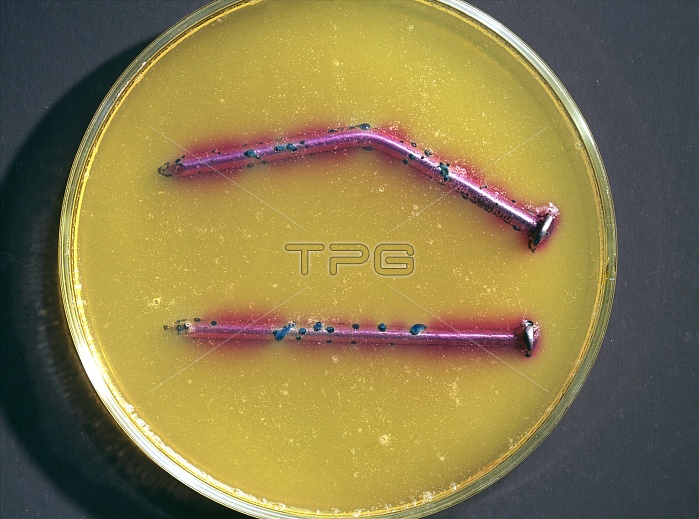
Corrosion test. Nails being tested for corrosion (blue spots). They are in a petri dish containing agar (brown). A chemical indicator called ferroxyl has been used to test for the presence of the ions produced during rusting. Ferroxyl indicator is a mixture of the acid-base indicator phenolphthalein and of potassium hexacyanoferrate. During rusting, the iron metal of the nails is oxidised and loses electrons to form iron ions. These react with the hexacyanoferrate ion to form the prussian blue ion that is an intense blue colour. The pink colour is where the phenolphthalein detects hydroxide ions (an alkaline solution) where no rusting occurs.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP10233681
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading