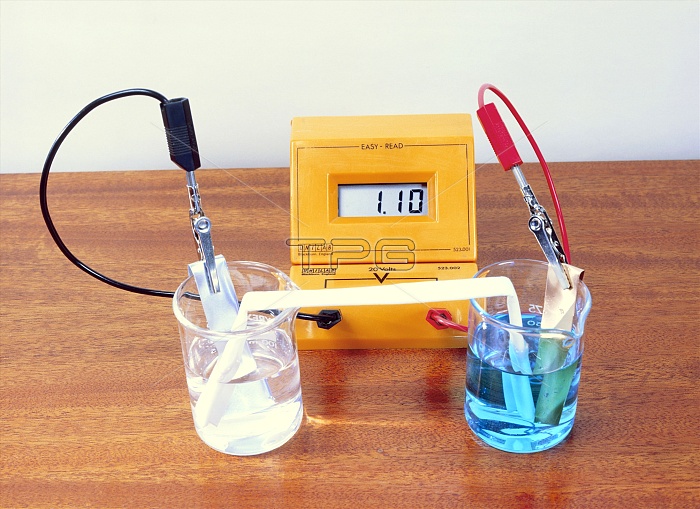
Zinc-copper battery. Voltmeter (orange) measuring a voltage (potential difference) of 1.1 volts for a zinc-copper battery cell. The cell consists of 2 strips of different metals dipped in electrolyte solutions (in beakers) and connected by a 'salt bridge' (white). On the right, copper is dipped in a copper sulphate solution, and on the left zinc is dipped in sulphuric acid. The zinc has a lower affinity for electrons than copper, and zinc from the zinc strip forms ions and electrons flow along the electrical wires and pull copper ions out of solution to form solid copper on the copper strip. Ion flow along the salt bridge closes the circuit.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP10163928
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading