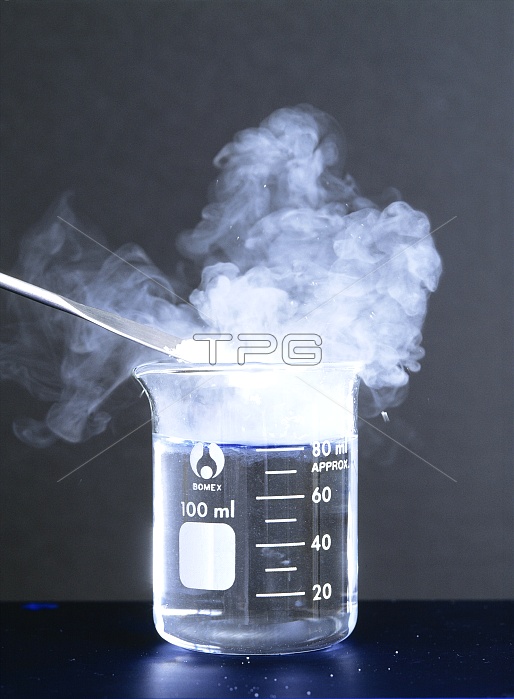
Phosphorus (III) chloride reacting with water. Chemical reaction between phosphorus (III) chloride (or phosphorus trichloride, PCl3) and water. With excess water, the violent reaction gives off fumes of hydrochloric acid (HCl) and phosphorous acid (H3PO3). The equation for this reaction is: PCl3 + 3H20 ----> 3HCl + H3PO3 Phosphorus (III) chloride is used in the chemical industry as a raw material in the manufacture of organophosphorus pesticides and other compounds, and as a chlorinating agent. It is a precursor chemical for the manufacture of some nerve agents, such as sarin.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP10163862
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading