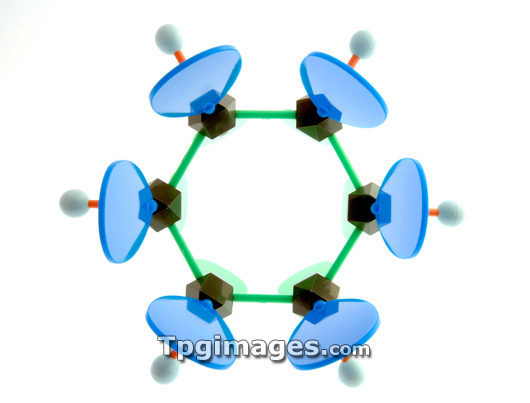
Benzene. Molecular model of the aromatic hydrocarbon benzene. The molecule is a flat (planar) hexagonal ring of six carbon atoms (black) with six hydrogen atoms (white) attached to the ring, one at each vertice of the hexagon. The chemical formula is C6.H6. The planar ring structure of bezene, where the carbon-carbon bonds are all of the same length, is explained by atomic orbital theory. The electron p-orbitals of the carbon atoms (green and blue hourglass shapes) protrude above and below the plane of the ring, but the electrons delocalise to form a circular pi-bond around the ring (delocalisation not shown here). This explains many of benzene's properties.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP03198397
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:

 Loading
Loading